Hoe goed werkt uw vaccin?
Vaccineren is een belangrijke stap op weg naar meer bewegingsvrijheid
De…
Innatoss and Erasmus MC enter into new cooperation
Researchers from Erasmus MC and Innatoss Laboratories in Oss…
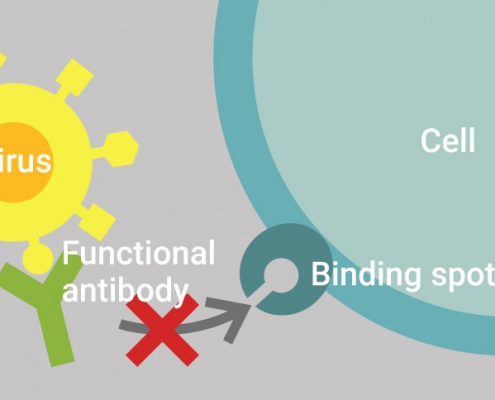
Innatoss first laboratory to Launch GenScript’s cPass™ test in Europe
Innatoss Laboratories first to Launch Neutralizing Antibody Public…
 https://www.innatoss.com/wp-content/uploads/Q-detect-productfotos-2.jpg
400
400
Saskia Theunisse
https://www.innatoss.com/wp-content/uploads/logo-InnatOss-groen.png
Saskia Theunisse2018-04-24 08:37:372018-05-24 10:01:34Q-detect™ comes out on top in Australian research
https://www.innatoss.com/wp-content/uploads/Q-detect-productfotos-2.jpg
400
400
Saskia Theunisse
https://www.innatoss.com/wp-content/uploads/logo-InnatOss-groen.png
Saskia Theunisse2018-04-24 08:37:372018-05-24 10:01:34Q-detect™ comes out on top in Australian research https://www.innatoss.com/wp-content/uploads/Naamloos-1-4.jpg
500
500
Saskia Theunisse
https://www.innatoss.com/wp-content/uploads/logo-InnatOss-groen.png
Saskia Theunisse2018-03-06 15:11:182018-05-24 10:01:35Chronic q fever from a sledge dog or seal, are you serious?
https://www.innatoss.com/wp-content/uploads/Naamloos-1-4.jpg
500
500
Saskia Theunisse
https://www.innatoss.com/wp-content/uploads/logo-InnatOss-groen.png
Saskia Theunisse2018-03-06 15:11:182018-05-24 10:01:35Chronic q fever from a sledge dog or seal, are you serious?
New research shows persistent Lyme infection in rhesus monkeys
Research done by the Tulane University (New Orleans, LO, USA)…
 https://www.innatoss.com/wp-content/uploads/shutterstock_103937081.jpg
667
1000
Saskia Theunisse
https://www.innatoss.com/wp-content/uploads/logo-InnatOss-groen.png
Saskia Theunisse2018-02-09 10:00:112018-05-24 10:01:35Tick carries more than just Lyme disease
https://www.innatoss.com/wp-content/uploads/shutterstock_103937081.jpg
667
1000
Saskia Theunisse
https://www.innatoss.com/wp-content/uploads/logo-InnatOss-groen.png
Saskia Theunisse2018-02-09 10:00:112018-05-24 10:01:35Tick carries more than just Lyme disease https://www.innatoss.com/wp-content/uploads/afbeelding-dracula-teek.jpg
582
685
Saskia Theunisse
https://www.innatoss.com/wp-content/uploads/logo-InnatOss-groen.png
Saskia Theunisse2018-02-02 09:00:542018-05-24 10:01:35Meet Dracula, the dinosaurs’ tick
https://www.innatoss.com/wp-content/uploads/afbeelding-dracula-teek.jpg
582
685
Saskia Theunisse
https://www.innatoss.com/wp-content/uploads/logo-InnatOss-groen.png
Saskia Theunisse2018-02-02 09:00:542018-05-24 10:01:35Meet Dracula, the dinosaurs’ tick
Innatoss and MediSapiens Receive EU Grant to Personalize Lyme Diagnostics
Innatoss and MediSapiens have partnered to develop a novel Lyme…

Innatoss only Dutch participant in quality research
In order to research the quality of our work, Innatoss participates…
